Enthalpy Change of Atomisation
It means Enthalpy is the energy that break one Mole of bond into Atoms. CH4 g C g 4H g.

For Part I How Do I Write The Equation For Atomisation I Remember In Enthalpy Change Of Atomisation One Mole Of Atom Is Formed From Substance That Is In Its Standard State
29 Jul 07 2220.

. Thats why the standard enthalpy change of atomisation for bromine is a conversion from liquid to gaseous state. Pg60 This relates the enthalpy of formation of an Ionic solid to the enthalpies of atomisation of the elements concerned the ionisation. For All the diatomic molecules Ex- Cl2 O2 their enthalpy of atomization will be the same as bond dissociation energy.
Enthalpy of atomization Δ a H 0 is the change in enthalpy when one mole of bonds is completely broken to obtain atoms in the gas phase. 1 This is often represented by the symbol H or HAll bonds in the compound are broken in atomization and none are formed so enthalpies of atomization are. Change in enthalpy Δ H Δ U Δ PV Change in enthalpy Δ H Δ U P 2 V 2 P 1 V 1 Change in enthalpy Δ H 30 20 6 Change in enthalpy Δ H 44 L atm.
The Enthalpy of Atomization is the change in Enthalpy that accompanies the total separation of all Atoms in a chemical substance either a Chemical Element or a Chemical Compound. So c6h5n s 6C g 5H g N g b unclebutcher. The standard enthalpy change of atomisation of a compound symbol delta H atm with the circle with line thru it is the enthalpy increase that takes place when one mole of the compound in the defined physical state is broken down into gas atoms under standard conditions.
ΔHa 16650 kJ per mol For diat Continue Reading More answers below Piyush Jena. The standard enthalpy change of atomisation is the enthalpy change when one mole of gaseous atoms is formed from its elements under standard conditions now under standard conditions bromine is a liquid. CH 4 g C g 4 H g Ha 0 16650 kJ mol-1.
Enthalpy of atomisation ΔHa is the change in enthalpy when one mole of bonds are completely broken to obtain atoms in the gas phase. The reaction of cyanamide NH 2 CN s with dioxygen was carried out in a bomb calorimeter and U was found to be 7427 kJ mol 1 at 298 K. Which equation represents the change corresponding to the enthalpy change of atomisation of iodine.
A 12 I2g Ig B I2g 2Ig C 12 I2s. Bond dissociation enthalpy is the enthalpy change for a mole of substance to break its covalent bonds into its atoms in a gaseous state. Atomization of gas molecules.
Enthalpy of atomization is the modification in physical property once one mole of bonds is totally broken to get atoms within the gas part. You must include the change in energy when an atom loses an electron ionisation energy and the change in energy when an atom gains an electron electron affinity as individual steps in a Born-Haber. This is dependent on the standard enthalpy and entropy changes and the temperature.
Polyatomic molecules- For polyatomic molecules the above is not true. It IS vaporisation I. Atomization of methane molecule.
The enthalpy of atomization also atomisation in British english is the enthalpy change that accompanies the total separation of all atoms in a chemical substance either a chemical element or a chemical compound. J Enthalpy of atomisation AH is the enthalpy change for the production of one mole of atoms in the gas phase from the element in its standard state. Ithe reaction of magnesium with hydrochloric acid.
It is always endothermic. ΔHa 16650 kJ per mol For diat Continue Reading Priyanka Kumari. Enthalpy of atomization is denoted by the symbol ΔHa.
Atomization of methane molecule. The standard enthalpy of atomisation is the enthalpy change when one mole of gaseous atoms is formed from its element in its standard state. Enthalpy change Δ H refers to the amount of heat energy transferred during a chemical reaction at a constant pressure Enthalpy change of atomisation The standard enthalpy change of atomisation Δ Hatꝋ is the enthalpy change when 1 mole of gaseous atoms is formed from its element under standard conditions.
CH4 g C g 4H g. Enthalpy of atomisation ΔHa is the change in enthalpy when one mole of bonds are completely broken to obtain atoms in the gas phase.
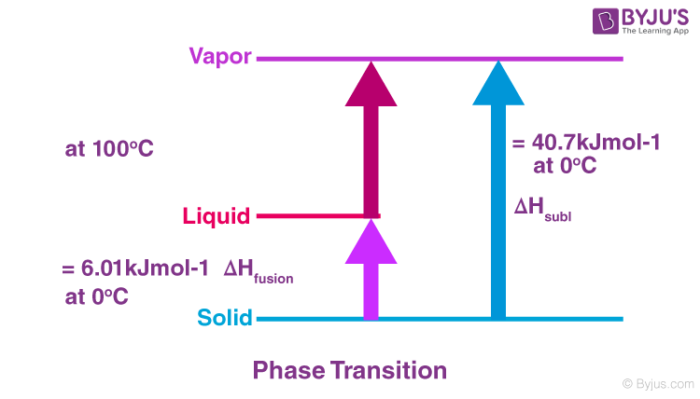
Enthalpy Of Atomisation Atomisation Of Transition Elements

78 57 Bond Enthalpy And Atomization Youtube
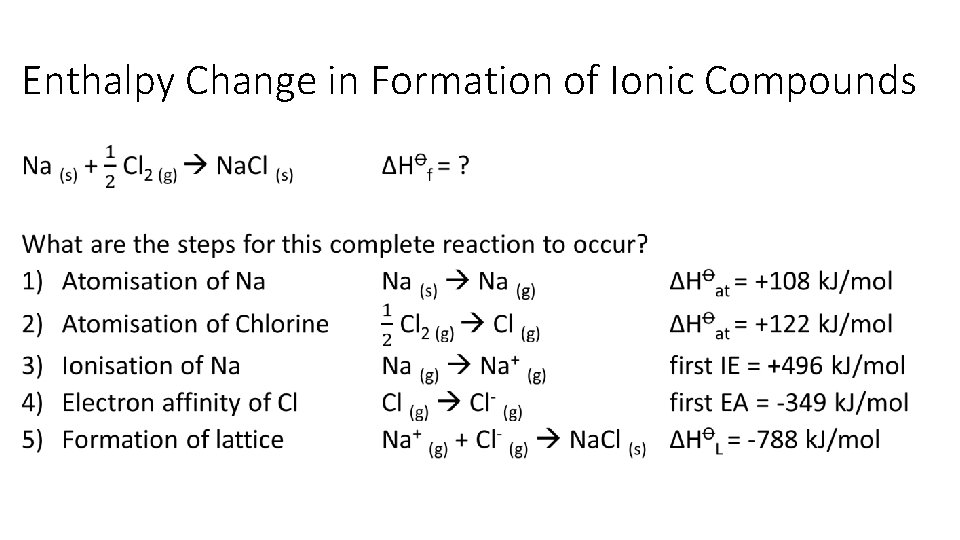
12 Thermodynamics 12 1 Types Of Enthalpy Change

Caie 9701 11 O N 20 Q 6 Enthalpy Changes Youtube

A Level Bond Enthalpy Bond Dissociation Energy Calculations For Enthalpy Of Reaction Ks5 Gce Chemistry Revision Notes
Comments
Post a Comment